In the last years, hydrogen has been considered as a promising energy vector for the oncoming modification of the current energy sector, mainly based on fossil fuels. Hydrogen can be produced from water with no significant pollutant emissions but in the nearest future its production from different hydrocarbon raw materials by thermochemical processes seems to be more feasible. In any case, a mixture of gaseous compounds containing hydrogen is produced, so a further purification step is needed to purify the hydrogen up to required levels accordingly to the final application, i.e., PEM fuel cells. In this mean, membrane technology is one of the available separation options, providing an efficient solution at reasonable cost. Particularly, dense palladium-based membranes have been proposed as an ideal chance in hydrogen purification due to the nearly complete hydrogen selectivity (ideally 100%), high thermal stability and mechanical resistance. Moreover, these membranes can be used in a membrane reactor, offering the possibility to combine both the chemical reaction for hydrogen production and the purification step in a unique device.
Hydrogen is advised as a very promising energy carrier due to its long-term viability, high energy density (14 J·kg-1·℃-1), environmentally welcoming combustion emissions and high resources to be produced from. Indeed, hydrogen is the most abundant element in the Earth, although it is usually found combined with other elements, mainly in water and hydrocarbon molecules. The idea is to transfer the energy obtained for different primary energy sources, preferentially renewables (i.e., wind, solar or biomass, among others), to hydrogen, which can be stored, transported and eventually used in different energy applications. Ideally, hydrogen will be obtained from water by using these renewable energies, thus minimizing the environmental impact while the energy demand is covered. However, the hydrogen generation by thermochemical processes seems to be a more realistic option in the near future for cost-cutting. Hence, hydrogen can be generated from a wide variety of raw materials containing hydrocarbons for both centralized and distributed production systems by using relatively mature technologies, being the use of biomass and waste materials especially attractive. In these cases, a mixture of gaseous compounds is frequently produced, being necessary to purify the hydrogen up to required levels accordingly to the final application, i.e., PEM fuel cells, turbines or combustion engines. Indeed, the hydrogen purification step is crucial process in the successful implementation of the hydrogen energy system from both technical and economical point of view.
Among readily available alternatives for hydrogen purification, the use of membranes for hydrogen separation/production applications has been proposed and used in practice. This technology shows relevant advantages such as low energy consumption, environmentally good properties and also additional potential to be combined with a reaction unit in a multifunctional membrane reactor. The combination of simultaneous chemical reaction and hydrogen separation in one unique step results in additional benefits in terms of conversion increase by shifting the reaction equilibrium as one of the products, hydrogen, is selectively separated from the reaction media. Particularly, dense metallic based membranes have been proposed for years due to their potential to transport hydrogen in a dissociative form with a theoretically complete perm-selectivity. Thus, the structure of metals belonged groups III-V, such as Pd, Ni and Pt (in pure form and alloyed), has the ability to allow the hydrogen diffusion through the metal lattice, while avoiding the permeation of other molecules. In this way, the solution-diffusion mechanism, depicted in Figure 1, is used to describe the hydrogen permeation process in these H2-selective membranes.
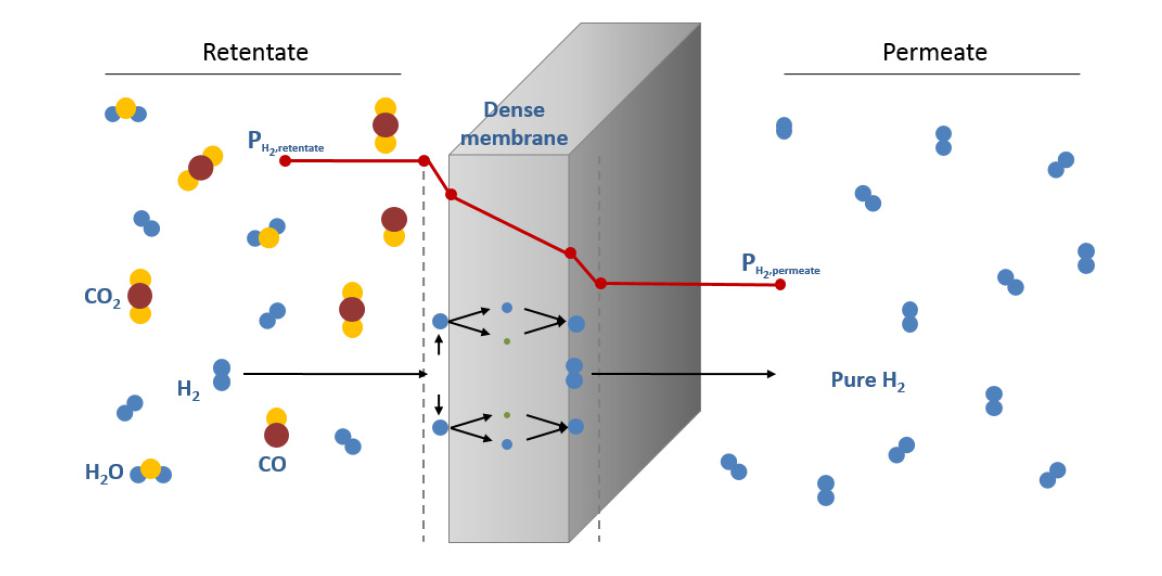
Figure 1. Solution-diffusion mechanism for hydrogen permeation through the metal lattice of a dense membrane.